Gametophytes for this species vary widely in morphology from relatively thin green filaments (Image A) coating the rocks (Featured Image above) to moniliform (like a string of beads) plants the cells of which can reach 2 mm in diameter (Image B). In both cases the individuals are dark green and can reach 4-10 (20 for the larger beaded individuals) cm in length. The erect filaments are typically formed in carpets that arise from a compact anchoring system of intertwined rhizoids (Image C). These descending rhizoids are produced from lower vegetative cells, in some individuals from as much as >1 mm away from the base (Image D). Below this point the axes, 52-60 µm wide, become dominated by descending rhizoids, which can mask the remnant main filament cells (Image E). At the very base where individual filaments attach the axes can be dominated by rhizoids (Image F) or the basal filament cell can be seen, but there are still subtending rhizoids (Image G). Although most rhizoids are intramatrical (i.e. produced within the “matrix” or cell walls and surrounding cuticle) (Images D & E), occasionally extramatrical (i.e. free descending rhizoids) are also produced (Image H). More rarely free ascending rhizoids are produced low on the filaments, the cells of which are similar to the erect filament cells (Image I). Vegetative cells near the filament base were typically rectangular, 28-47 µm wide by 50-210 µm long, occasionally square, at times atypically narrow and long, 27 µm by 265 µm (Image J). Mid filament cells were similarly shaped, 55-65 µm wide by 105-202 µm tall, although irregularities were again common (one cell was a disc shape, 60 µm wide by 25 µm tall; Image K). In terminal regions the cells started to bulge, being typically rectangular with a slight barrel appearance, 73-106 µm wide by 142-175 µm tall (Image L) (to 2 mm in diameter in moniliform individuals, Image B). Putative gametangia, 67-72 µm wide by 82-90 µm tall with a slight barrel shape, develop in the upper regions of the erect filaments and have apertures for zoid release (Image M). Throughout cell walls are thick and in fresh material a parietal chloroplast covers only part of the cell. The zygote is reportedly a ‘Codiolum pusillum‘ type stage that we have not yet encountered (for an example see Urospora neglecta (Kornmann) Lokhorst & Trask).
We have only a few (n = 3) genetically verified records, these from upper intertidal pools on rock to subtidal (3 m) from BC and NS. All collections are from the spring to early summer, but this is partly a sampling artifact (e.g. I have yet to collect the BC winter flora as part of our ongoing biodiversity surveys). Although a distinct species in its beaded morphology (Image B), which when I first collected it reminded me of Chaetomorpha darwinii (J.D.Hooker & Harvey) Kützing (= Chaetomorpha coliformis (Montagne) Kützing, but who wants to diminish a tribute to Darwin) from my time in Australia, this species can easily blend in with the rich diversity of other Urospora and Ulotrhix spp. and be overlooked in its filamentous morphology (Image A). In fact in our limited genetically verified collections the moniliform morphology (Image B) was collected only once in southern BC, while a collection each from Haida Gwaii and Nova Scotia were filamentous (Image A). We do have a moniliform collection from QC (Image N; GWS006166), but it returned rbcL data for Urospora penicilliformis (Roth) Areschoug. There were a few filaments more typical of the latter tangled among the large cells of GWS006166 and this sequence anomaly may represent PCR contamination. We are currently trying to obtain other markers from this collection to resolve this conundrum. More recently (2023) specimens sent by colleagues in Quebec with this morphology were assigned to U. wormskioldii based on ITS data (Image O) extending the moniliform morphology to QC.
More collections are needed from all regions to better understand this species’ morphological, ecological and biogeographical range. Finally, this species as well as all others in our flora assigned to Urospora, do not join the type species Urospora penicilliformis (Roth) Areschoug and taxonomic change at the genus level is also needed.

Image A. Dark green filaments scraped from an upper intertidal pool on rock (Black Rock, Bay of Fundy, NS: GWS013967).

Image B. Press of moniliform specimens, the morphology typically associated with this species (subtidal (3 m) on rock, Otter Point, BC; GWS006374).

Image C. Compact tangle of descending rhizoids form the extensive anchoring system for the carpet of erect filaments (GWS013967; rehydrated from silica vial).

Image D. Cells >1 mm (arrow) from the base can contribute to the descending rhizoids (GWS013967; rehydrated from silica vial).
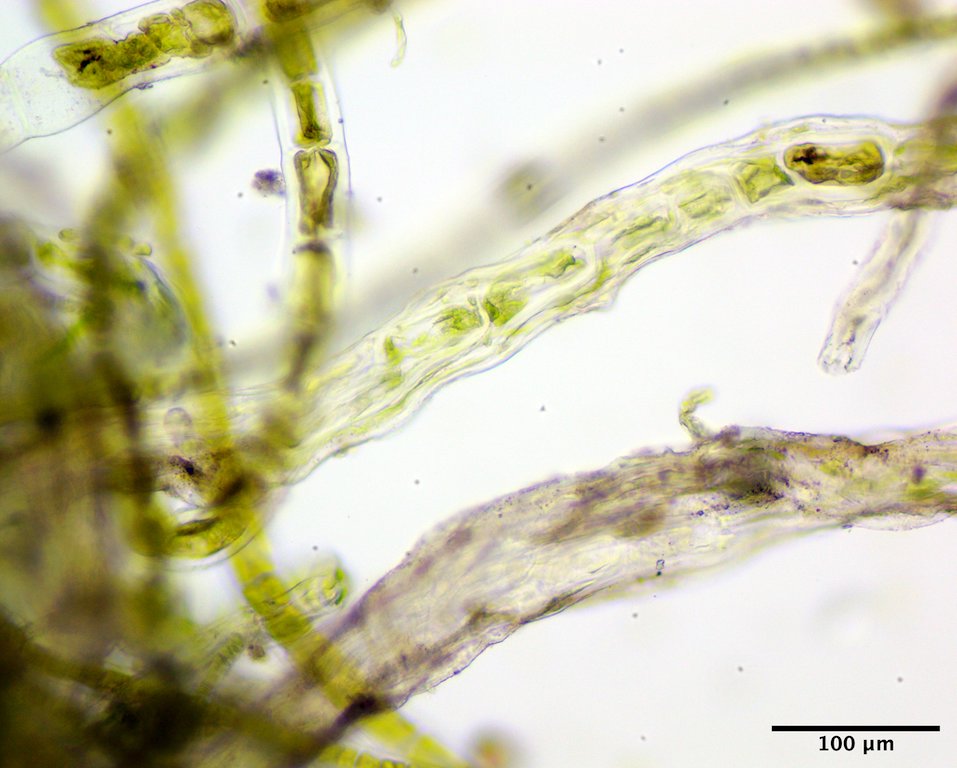
Image E. Near the base individual filaments become dominated by descending rhizoids (filament above) and eventually the main filament cells can become masked (filament below) (GWS013967; rehydrated from silica vial).

Image F. Individual plant removed from the compact holdfast revealing the anchoring tip of descending rhizoids (GWS013967; rehydrated from silica vial).

Image G. Basal cell of the erect filament evident in this individual, subtended by a rhizoid from a cell further up the filament (GWS013967; rehydrated from silica vial).
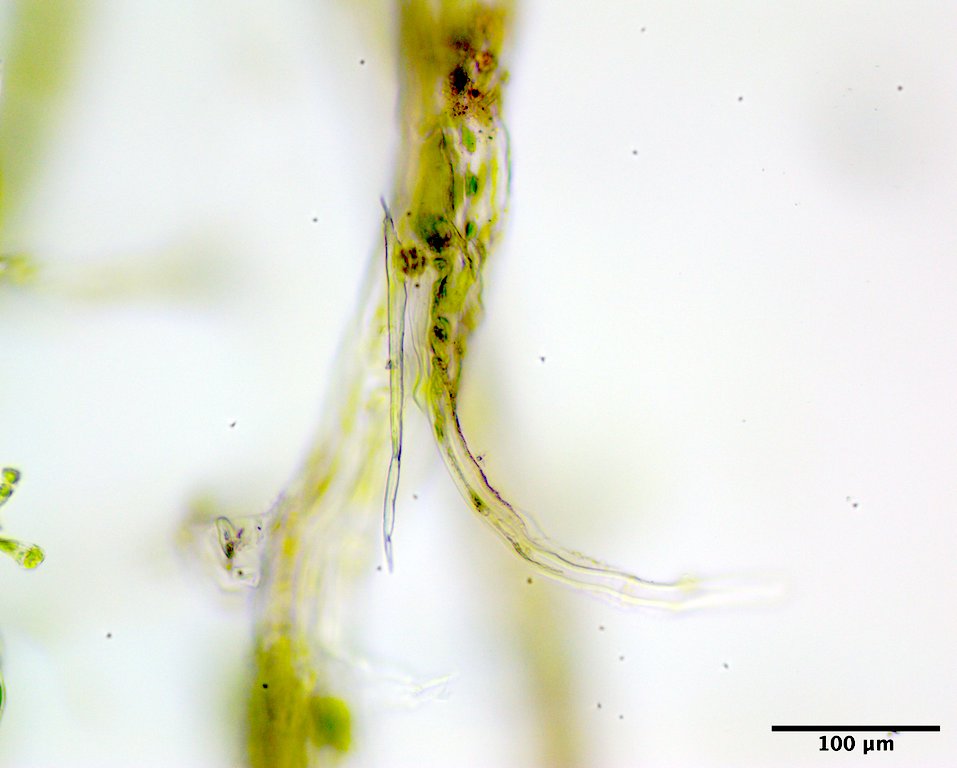
Image H. Extramatrical descending rhizoid produced from a filament cell near the base (GWS013967; rehydrated from silica vial).

Image I. Free ascending rhizoid-type filament produced low on the filament, but just above the descending rhizoid production with the cells similar in appearance to regular vegetative cells (GWS013967; rehydrated from silica vial).

Image J. Erect filament cells near the base were typically rectangular, at times atypically narrow and long (arrow), and occasionally square (GWS013967; rehydrated from silica vial).

Image K. Mid filament cells were typically rectangular, but with some exceptions (arrow) (GWS013967; rehydrated from silica vial).

Image L. Larger rectangular cells, slightly barrel-shaped, towards the top of the axes (GWS013967; rehydrated from silica vial).

Image M. Putative gametangia with developing apertures for zoid release (arrows) (GWS013967; rehydrated from silica vial).

Image N. Moniliform collection from low intertidal on cobble, Escoumins (east of town across from Rue aux Bouchets), QC (GWS006166).

Image O. Moniliform collection sent by Antoine Nicolas (image credit) and Éric Tamigneaux (for which I am very thankful) from the subtidal (1 m) on boulders, Cap-aux-Os, QC (GWS045197).
